Alzheimer’s Clinical Trials Consortium
The AHEAD 3-45 Study tests whether an investigational treatment can slow or stop the earliest brain changes due to Alzheimer’s disease (AD) in people with a higher risk of developing the disease later in life.
AD is an age-related neurodegenerative disorder characterized by progressive decline in cognitive function and the ability to perform activities of daily living, ultimately resulting in dementia with fatal complications.
The amyloid hypothesis of AD postulates that the accumulation of amyloid-β peptide (Aβ) is an early and necessary event in the pathogenesis of AD. This hypothesis suggests that treatments that slow the accumulation of Aβ in the brain or increase clearance of Aβ may be able to slow the progression of the AD clinical syndrome.
Converging evidence from both genetic at-risk and age at-risk cohorts suggests that the pathophysiological process of AD begins well more than a decade before the clinical stage now recognized as AD dementia, as a long asymptomatic or preclinical stage, and that neurodegeneration is already apparent by the stage of mild cognitive impairment (MCI)/prodromal AD.
Recent clinical trial results in mild-to-moderate AD dementia, as well as evidence from transgenic animal experiments, suggest that earlier intervention may be most beneficial to fully impact the clinical progression of the disease, particularly with therapies targeted at Aβ reduction.
Data from autopsy cohorts and cerebrospinal fluid (CSF) and positron emission tomography (PET) scan amyloid imaging studies demonstrate that approximately 30% of individuals over the age of 65 have evidence of amyloid pathology. Clinically “normal” older individuals with biomarker evidence of elevated amyloid pathology demonstrate AD-like abnormalities on functional and structural imaging, perform less well on cognitive tests compared to amyloid-negative older individuals, are more likely to report subjective memory concerns, and are at increased risk for cognitive decline and progression to MCI and AD.
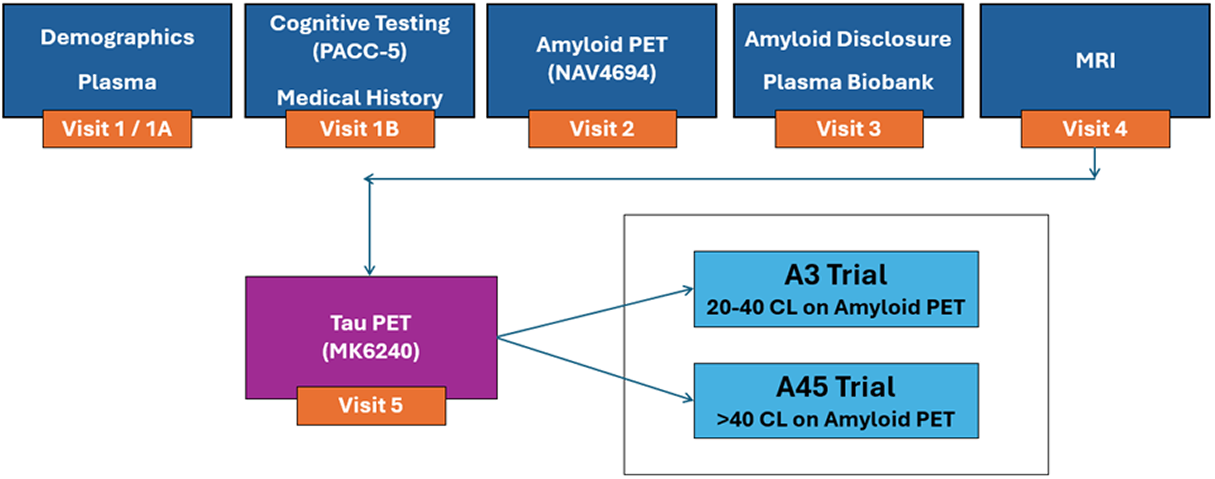
AHEAD 3-45 is a Phase 3, global, multicenter, double-blinded, placebo-controlled randomized clinical trial aimed at preventing memory loss due to AD in the asymptomatic stage of the AD continuum. It consists of two sister studies, A3 and A45, with distinct participant groups but which had shared screening and recruitment to increase efficiency.
The primary purpose of the AHEAD 3-45 Study is to determine whether treatment with Lecanemab (BAN2401) is superior to placebo on change from baseline of the Preclinical Alzheimer Cognitive Composite 5 (PACC5) at 216 weeks of treatment in A45, and to determine whether treatment with Lecanemab (BAN2401) is superior to placebo in reducing brain amyloid accumulation as measured by amyloid positron emission tomography (PET) at 216 weeks of treatment in A3.
Lecanemab (BAN2401) is a humanized immunoglobulin 1 (IgG1) monoclonal antibody that preferentially targets soluble aggregated Aβ. This study is also evaluating the long-term safety and tolerability of Lecanemab (BAN2401) in participants enrolled in the Extension Phase.
Learn more about the AHEAD 3-45 Study below.
Our Study
The AHEAD 3-45 screening dataset is the largest cohort of cognitively unimpaired older individuals with amyloid imaging and detailed cognitive testing available to date. The study was launched in July 2020 and was fully enrolled in 2024 with 20,720 total global screens. The study participants are cognitively unimpaired individuals between 55 and 80 years of age with evidence of intermediate (A3) or elevated (A45) amyloid plaque accumulation in the brain on screening PET.
Of the global screens, 16,835 screens are from North America research sites which is what this accessible dataset is limited in scope to at this time.
The AHEAD 3-45 Study has approximately 100 study locations worldwide, including in North America, Japan, Singapore, Australia, and Europe—more than 70 of which are in the United States and Canada.
A3 Study
The A3 Study aims to get closer to primary prevention of AD, through preventing amyloid build-up in the brain. The clinical trial targets cognitively normal individuals who show intermediate levels (20-40 centiloids) amyloid PET but are at high risk for further Aβ accumulation. A3 compares the effects of low dose Lecanemab (BAN2401) versus placebo, to test whether an anti-amyloid beta antibody targeted at protofibrils can slow brain amyloid accumulation at this very early stage of disease. The A3 Study is also measuring the accumulation of tangle pathology using tau PET scans and exploratory cognitive outcomes.
A45 Study
The A45 Study targets the later preclinical (pre-symptomatic) stage of AD. This clinical trial targets clinically normal participants (with little to no cognitive impairment) who have elevated levels of amyloid in brain (>40 centiloids) and are at high risk for cognitive decline, and eventual progression to mild cognitive impairment and AD dementia. A45 is looking at a treatment regimen consisting of an anti-amyloid beta antibody targeted at protofibrils to prevent cognitive decline and delay biomarkers of pathological progression versus placebo. In the active arm, individuals are treated with high-dose Lecanemab (BAN2401) to clear amyloid deposits and Aβ protofibrils from the brain, followed by low-dose Lecanemab (BAN2401) to prevent re-accumulation of amyloid. The aim of the A45 clinical trial is to slow or prevent decline in cognitive performance.

Study Leadership
The AHEAD 3-45 Study is led by AD research experts and academic leadership at the University of Southern California's Alzheimer's Therapeutic Research Institute (ATRI), Bringham and Women's Hospital, Massachusetts General Hospital, and Harvard Medical School.
Partnerships and Funders
The AHEAD 3-45 Study is conducted as a public-private partnership of the Alzheimer's Clinical Trial Consortium (ACTC), funded by the National Institute on Aging (NIA) at the National Institutes of Health (NIH), and Eisai Inc.
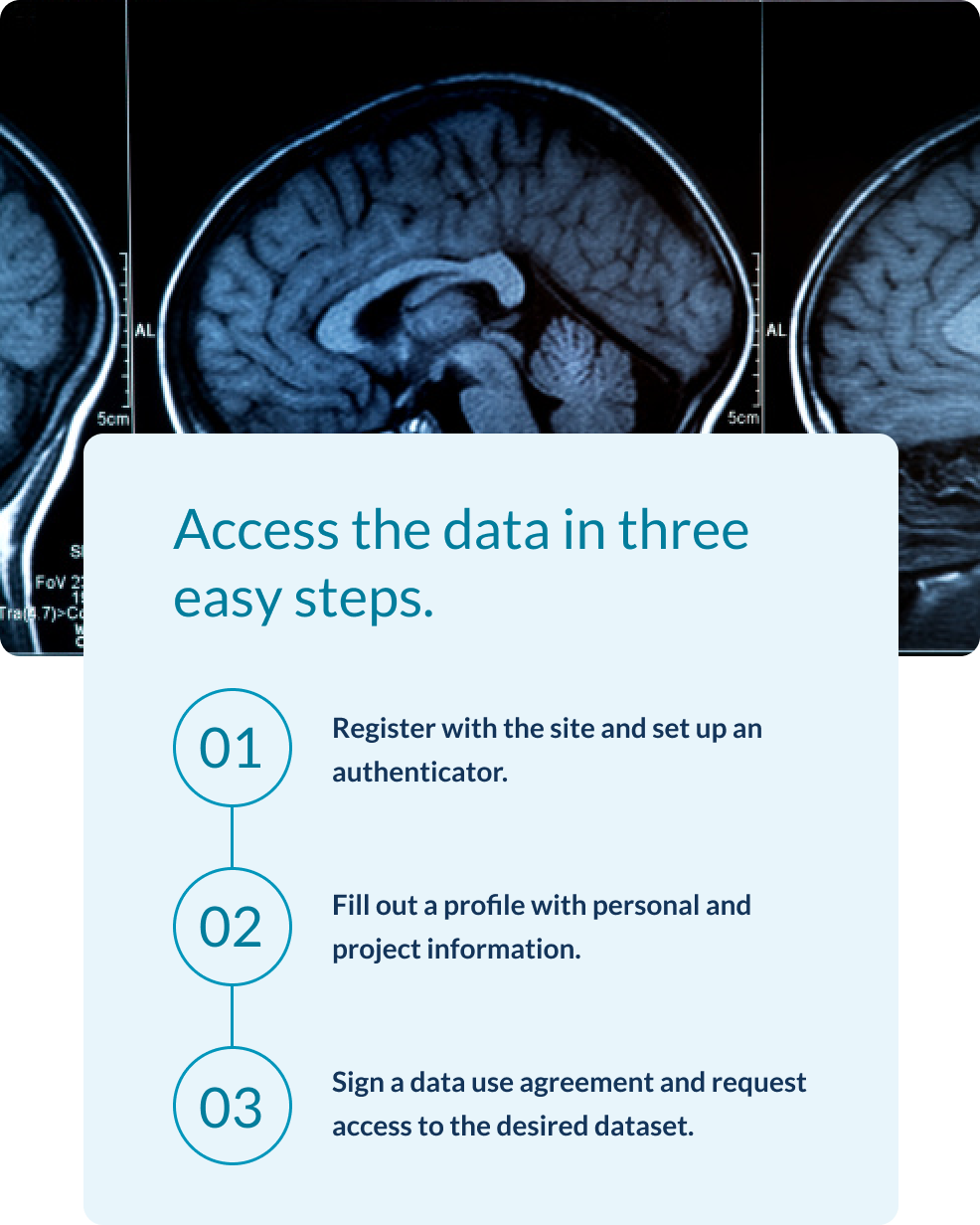
AHEAD 3-45 pre-randomization data are being made available through this website within one year after completion of all participant randomizations. The accessible data is limited to screening data only at this time and for AHEAD 3-45 research sites within the United States and Canada (North America) due to data sharing regulations of other countries.
We wish to express immense gratitude to the hundreds of AHEAD 3-45 participants and their study partners across the world, and to the dedicated AHEAD 3-45 research teams across many institutions as this study would not be possible without their continued dedication to the study.
Discover the AHEAD 3-45 Study research
April 19, 2023
The AHEAD 3-45 Study: Design of a prevention trial for Alzheimer's disease
Frequently asked questions
For additional information, please contact us.
For general inquiries, please contact us. For data requests, kindly register on our platform.

Alzheimer's Clinical Trials Consortium
The ACTC designed and is conducting the AHEAD 3-45 trial. The ACTC lead the various novel components of the trial including the addition of plasma prescreening to reduce participant burden, as well as other recruitment approaches aiming to improve inclusivity of the trial. Even before enrollment was complete, methods and learnings from the pre-randomization data had been published, showing differences in racial in ethnic groups in plasma amyloid eligibility. Data sharing for this study will be a significant contribution to the field of AD research and all pre-randomization data and imaging studies are being shared in 2025 through this website.
ACTC’s mission is to provide an optimal infrastructure, utilizing centralized resources and shared expertise, to accelerate the development of effective interventions for AD and related disorders.